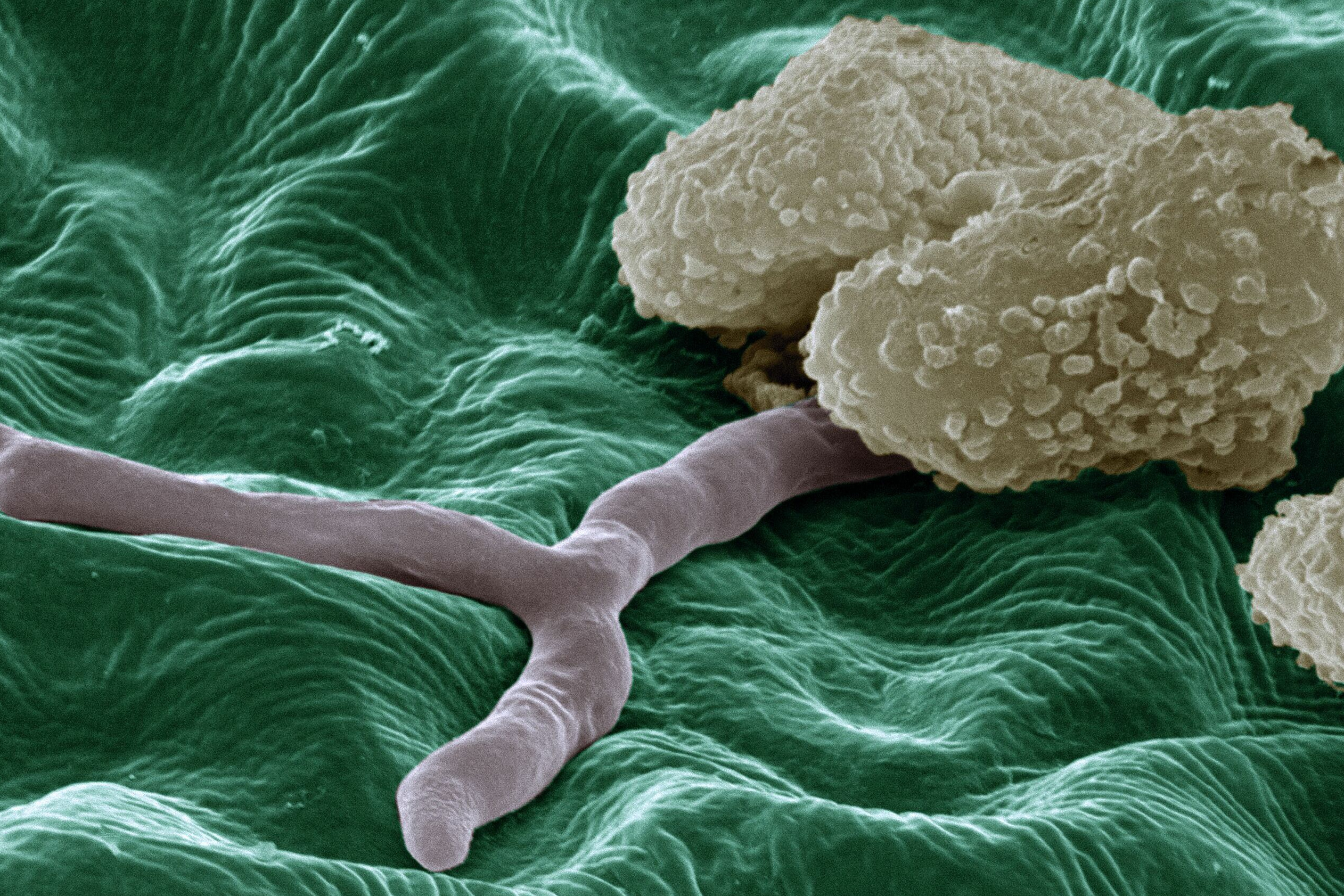
Communication between plants and fungi

Within a project funded by the German Research Foundation (DFG), molecular biologist Vera Göhre from h_da (Darmstadt University of Applied Sciences) is examining how harmful fungi communicate with their host plants. Specifically, her work focuses on fungi that infest crops such as maize, cabbage, beet, rice or rape. Göhre and her team want to find out exactly how they manage to penetrate their “hosts” and reprogramme them. Here, devious communication channels are at play that scientists are only gradually beginning to understand. The findings could make an important contribution to global nutrition: several hundred fungal diseases are known worldwide, which, according to estimates by the Max Planck Society, lead to crop failures of twenty to forty percent every year. A development exacerbated by climate change and increasing resistance.
By Christina Janssen, 13.12.2024
Weeding is not necessarily part of a professor’s remit. Or are things different at h_da? In any case, one of the first things that molecular biologist Professor Vera Göhre did upon her arrival in Darmstadt in the spring was to grab one of the concrete tubs on campus and plant rockcress seedlings in it. Since then, she has nurtured and cultivated them – and made them part of her courses. What’s special about her seedlings is that some are infected with the smut fungus Thecaphora thlaspeos while others are not. In her lecture, she shows her students the wispy, grey-green shoots. “But none of them is able to distinguish between infected and uninfected plants,” she reports. This is not because the students are incompetent, but because the fungi are cunning and only reveal their true faces at a late stage when the plant is already producing seeds.
As crafty as Odysseus
Odysseus and his Trojan horse almost pale in comparison to the ingenuity of the fungus. Step 1: The fungal spore lands on its host plant and “recognises” its potential prey. Step 2: The spore germinates. Step 3 is where it gets tricky: the fungus must somehow enter the plant. The tough cell wall makes this extremely difficult. Once the fungus has succeeded, Step 4 follows: the fungus smuggles “saboteurs” into the plant cell in the shape of small molecules – and the point of no return is reached: once inside, the fungus reprogrammes the plant cell. From now on, the cell zealously produces nutrients to feed the parasite. How exactly this happens still puzzles the scientific community.
“How do fungi feed inside plants? How do they modify plants to their own advantage? And how do plants react to this?” says Vera Göhre, describing her main research questions. Whoever can answer them will one day be able to prevent fungal infestation without the use of toxic fungicides. That’s the hope. In April, Göhre was appointed as Professor for Molecular Biotechnology and Functional Genomics at h_da. Prior to this, she conducted research for twelve years at Heinrich Heine University Düsseldorf, where she was also group leader in the Cluster of Excellence for Plant Sciences (CEPLAS) funded by the German Research Foundation (DFG). In the project she brought with her to Darmstadt, Göhre wants to get to the bottom of the complex relationship between harmful fungi and plants.
Matchmaking for mushrooms?
It all began with a “date” between Göhre and the perfect fungus: in a witty article for the CEPLAS cluster, she described her search for the best possible research object as a kind of “matchmaking for mushrooms”. The smut fungus Thecaphora thlaspeos was the “chosen one”. Since then, Göhre and her team have already found out a lot about it, not over a candlelight supper but in the laboratory. One of the big puzzles is Step 3: How does the fungus enter the plant? “There are no doors in the cell wall,” says Göhre succinctly, knocking on her office wall. “If I want to visit my colleagues in the office next door, I go out through my door and in through theirs. But if I’m a fungus and this wall here is a plant cell, I have to drill a hole in it. And that’s exactly what the fungi do.”
To do this, the cunning fungi delve into their bag of molecular biology tricks: “For example, they can secrete enzymes that ‘eat away’ the cell wall.” Behind it, the fungus comes up against the cell membrane, which is permeable for some substances, such as sugar molecules that the plant produces in its cells during photosynthesis. “The plant cell happily releases these to the outside, ‘assuming’ that they will be transported to the roots or flowers. But if the fungus has just ensconced itself there, it can simply harvest the sugar.”
Groundbreaking finding
But that is not enough to satisfy the pesky fungus: it additionally channels its own proteins and genetic information through the membrane into the plant cells. “The new scientific approach is to examine this ‘skin contact’ between the fungus and the plant. In other words, to find out what happens when the fungal membrane and the cell membrane come together,” explains Göhre. “We know from medicine that pathogens systematically pack certain molecules into small ‘bubbles’ called vesicles.” According to the latest research, fungi also use such vesicles to smuggle their “tools” into plant cells. This is a groundbreaking finding: “Up until three or four years ago, scientists obstinately maintained that this was impossible, that nothing could enter or exit through the tough wall of a plant cell.” In the meantime, the prevailing opinion is that “Trojan” vesicles do indeed exist. “As part of the exRNA Research Unit, we are currently working with our colleagues in Düsseldorf to prove conclusively for corn smut that the same thing is found inside these vesicles again and again and that it has a function in the plant cell.” RNA and proteins, for example, which the fungus uses to switch off the plant’s immune system and take control of the cell nucleus, the cell’s control centre.
And what about the plants? They by all means attempt to defend themselves. They, too, can release small RNA “bubbles”. A battle breaks out between fungus and plant vesicles. And it is precisely here that the researchers want to take a closer look: “If we can identify the RNAs that the plant dispatches to defend itself, we can use them for plant protection, either by genetically modifying the plants in such a way that they produce more of them by themselves or by developing RNA sprays that also offer plant protection.” This is called spray-induced gene silencing, i.e. switching off genes (in the harmful fungus) with an RNA spray. The first start-ups in this area have already been established, reports Göhre. Such technologies could make the mass use of fungicides obsolete.
Many unanswered questions
But there is a long way to go. “There are still so many unanswered questions – more than I will be able to answer in my entire career,” says Göhre. “Just one example: How do the vesicles know the way? I think of it as a ball pit with red and blue balls – the red ones have to go from the fungus to the plant and the blue ones in the other direction. Total chaos. How does that work? That’s what I’d like to find out.” Together, a consortium with over ten research groups is looking for answers: How does a fungus know what to pack into its vesicles? Where exactly does this occur? How does a protein or an RNA know: I should or shouldn’t enter the vesicle, what signals are there for this? “Our research is still in its infancy,” says Göhre in conclusion.
The scientist, who worked in France, the USA, Australia, England and Switzerland before joining h_da, chose Thecaphora thlaspeos because it is genetically very simple. Just like the rockcress it infests. A “dream team” for research. “Each fungus has its plant, and each plant sometimes even has several fungi.” That is why Göhre’s project aims to produce transferable results that can also be applied to economically important fungi such as corn smut, grain smut and moulds. The overarching objective is better plant protection and higher yields – without using toxins. Or to make a virtue out of necessity: “In Mexico,” reports Göhre, who has travelled extensively, “cobs infested with corn smut are served as a delicacy.” And she finds rice blast, which has been cultivated in wild rice in China for 2,000 years as “water bamboo”, absolutely delicious. “Like a cross between mushroom and bamboo – really tasty. Perhaps the superfood of the future?” Her suggestion is not entirely serious, but anyone who starts their research project by dating a fungus clearly not only has bright ideas in the lab but also a sense of humour.
Contact the h_da science editorial team
Christina Janssen
Science Editor
University Communication
Tel.: +49.6151.533-60112
Email: christina.janssen@h-da.de
Translation: Sharon Oranski
Links
Sources
Max Planck Society, 9.10.2024:
Key facts on the impact of fungal infestations on global food security